Supported by the EuroIntervention Journal
This EIJ image highlights a new device designed to enhance the safety and efficiency of ViV TAVI procedures, particularly TAV-in-TAV, by reducing attempts to cross the valve and preventing guidewire misnavigation, thereby minimizing complications and procedure time.
Authors
Marco B Ancona, Ciro Vella, Vittorio Romano, Luca Ferri, Filippo Russo, Barbara Bellini, Matteo Montorfano
Case Summary
A crucial step in transcatheter valve-in-valve implantation (ViV TAVI) is the crossing of the bioprosthesis. The guidewire path, however, is hardly predictable, as the wire must navigate through a modified environment, which can include dilated aortic sinuses, bioprosthesis stents and frames, struts, or perivalvular leaks (PVLs), leading to a challenging and/or dangerous procedure1.
The EasyCrossTM device (ViVHeart, Milan, Italy) is a 12 Fr temporary catheter made by Pebax® polymers to maintain an optimal combination of toughness and flexibility. Its distal part is able to expand like a “basket” with 6 arms, by mechanical traction of the proximal part, allowing a three-dimensional distancing of the catheter from the aortic wall through a complete modulable range by pulling. Inside the device, it is possible to advance materials ranging from guidewire to 5 Fr catheter, with the aim of following a correct path across the bioprosthesis. The device is expected to reduce attempts to cross the valve, and to improve the safety of the procedure, avoiding the navigation of the guidewire through the wrong pathways (Figure panels A and B).
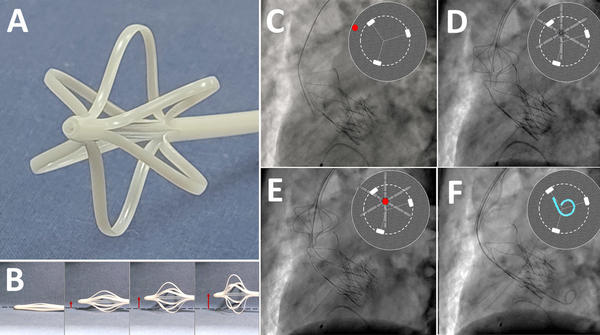
Panel A shows the EasyCrossTM device, a 12 Fr temporary catheter with a terminal part able to expand a distal “basket” with 6 arms to allow distancing from the aortic wall.
Panel B highlights the ability of the “basket” to expand three-dimensionally in a modulable range (red arrows).
Panel C shows a standard advancement of the guidewire, passing between the arches and the aortic wall (red dot in the reconstruction). The EasyCrossTM device was advanced and then expanded, distancing from the aortic wall, aligned with the bioprosthesis (Panel D) and permitting a safe and easy crossing of the outflow of the valve (Panel E – red dot). Finally, a pigtail was placed inside the device and then in the left ventricle (Panel F).
Due to its peculiar design, which includes large stabilization arches, one of the most challenging bioprostheses to cross is the Acurate Neo 2 (Boston Scientific, US)2,3,4.
An 84-year-old male with severe aortic stenosis, symptomatic of exertional dyspnea, was treated with percutaneous Acurate Neo 2 implantation. The patient was enrolled in the VIV-FIH (VIVheart transcatheter aortic valve replacement self-centering catheter First-in-Human feasibility – NCT06412354) clinical trial.
After the valve replacement, a 0.035’ J tip wire was advanced as expected outside the valve frame, following the aortic curve. Thus, the EasyCrossTM device was advanced in the ascending aorta, and the “basket” was open until reaching a desirable distance from the aortic wall to align with the bioprosthesis.
Then, the valve was safely crossed on the first attempt with the 0.035 J wire placed inside the EasyCrossTM (Figure panel E) and a pigtail catheter was safely and easily advanced in the left ventricle (Figure panels C to F; video 1 for rotational reconstruction and bench test).
Video 1: Rotational fluoroscopy (on the left) and bench test (on the right) showing how the pigtail passes safely inside the bioprosthesis through the Easycross™ device.
With the increasing number of ViV TAVI procedures in the future, especially TAV-in-TAV, where bioprostheses generally present specific designs and a higher rate of PVLs1, a dedicated device for successful and safe crossing could play a pivotal role in facilitating the already challenging procedure, while avoiding dangerous complications, and potentially reducing the duration of the procedure itself.
References
- Vella C, Romano V, Di Maio S, Ancona MB, Castriota F, Vassileva A, Ferri L, Bellini B, Moroni F, Russo F, Ghizzoni G, Gentile D, Palmisano A, Agricola E, Esposito A, Chieffo A, Montorfano M. Valve-in-valve transcatheter aortic valve implantation: The issues behind crossing a bioprosthesis. Cardiovasc Revasc Med. 2024 May;62:85-94. doi: 10.1016/j.carrev.2023.12.015. Epub 2023 Dec 26. PMID: 38160130.
- Vella C, Romano V, Ancona MB, Botti G, Ferri L, Russo F, Bellini B, Ghizzoni G, Beneduce A, Montorfano M. Incorrect Recrossing of Transcatheter Aortic Valve: A Dangerous Complication and its Effective Management. JACC Cardiovasc Interv. 2022 Aug 8;15(15):e173-e174. doi: 10.1016/j.jcin.2022.04.015. Epub 2022 Jun 15. PMID: 35926927.
- Kim WK, Hengstenberg C, Hilker M, Schäfer U, Rudolph TK, Toggweiler S, Rück A, Søndergaard L, Conradi L, Hamm C, Walther T, Möllmann H; Collaborators. Transcatheter aortic valve implantation with the ACURATE neo valve: indications, procedural aspects and clinical outcomes. EuroIntervention. 2020 Apr 3;15(18):e1571-e1579. doi: 10.4244/EIJ-D-19-00908. PMID: 31911402.
- Vanhaverbeke M, Kim WK, Mylotte D, Bieliauskas G, Janarthanan S, Sondergaard L, De Backer O. Procedural considerations for transcatheter aortic valve-in-valve implantation in a degenerated ACURATE neo prosthesis. EuroIntervention. 2023 Apr 24;18(17):1436-1438. doi: 10.4244/EIJ-D-22-00740. PMID: 36520089; PMCID: PMC10111127.
*Affiliations
- Interventional Cardiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- School of Medicine, Vita-Salute San Raffaele University, Milan, Italy.
Conflicts of interest
- Matteo Montorfano received consultant fees from Abbott, Boston, Kardia and Medtronic, and he is the President of ViVHeart
- Marco B Ancona received consultant fees from Abbott and Abiomed
Original Article
Read the original Article on PCR Online

